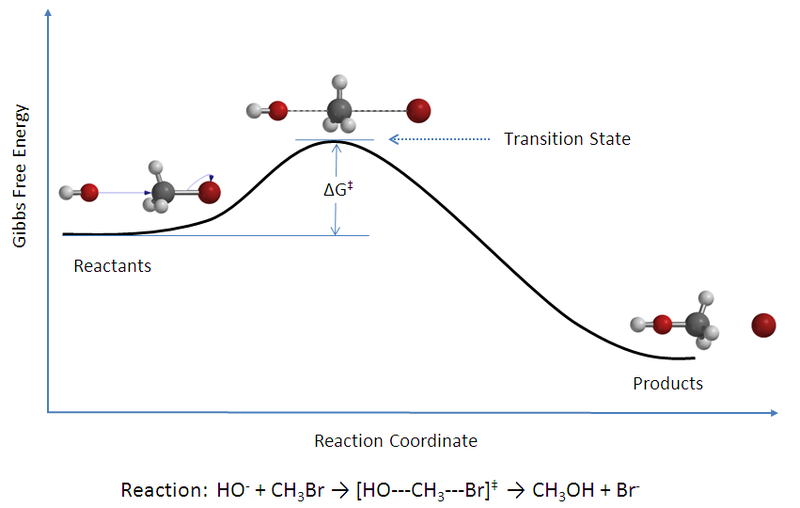
Differentiate Between Reactants And Products - By convention, scientists place the chemicals involved in a reaction into two basic categories: Products are substances that are produced in the reaction. Reactants and products are the two major components of a chemical reaction. The primary difference between reactants and products is that reactants are consumed during the reaction, whereas products are produced as a result of the reaction. Products are the chemical species. Reactants are substances that start a chemical reaction. This helps to explain what is happening during a reaction,. Reactants are the starting material of a chemical reaction. When a candle burns, the reactants are fuel (the candlewick and wax).
Reactants and products are the two major components of a chemical reaction. Products are the chemical species. Reactants are substances that start a chemical reaction. Reactants are the starting material of a chemical reaction. This helps to explain what is happening during a reaction,. When a candle burns, the reactants are fuel (the candlewick and wax). The primary difference between reactants and products is that reactants are consumed during the reaction, whereas products are produced as a result of the reaction. By convention, scientists place the chemicals involved in a reaction into two basic categories: Products are substances that are produced in the reaction.
Reactants are the starting material of a chemical reaction. This helps to explain what is happening during a reaction,. By convention, scientists place the chemicals involved in a reaction into two basic categories: When a candle burns, the reactants are fuel (the candlewick and wax). Products are substances that are produced in the reaction. Reactants are substances that start a chemical reaction. Reactants and products are the two major components of a chemical reaction. The primary difference between reactants and products is that reactants are consumed during the reaction, whereas products are produced as a result of the reaction. Products are the chemical species.
Difference Between Reactants and Products Definition, Properties
The primary difference between reactants and products is that reactants are consumed during the reaction, whereas products are produced as a result of the reaction. This helps to explain what is happening during a reaction,. Products are substances that are produced in the reaction. Products are the chemical species. When a candle burns, the reactants are fuel (the candlewick and.
Reactants vs. Products — What’s the Difference?
Products are the chemical species. The primary difference between reactants and products is that reactants are consumed during the reaction, whereas products are produced as a result of the reaction. When a candle burns, the reactants are fuel (the candlewick and wax). By convention, scientists place the chemicals involved in a reaction into two basic categories: This helps to explain.
What Is a Reactant in Chemistry? Definition and Examples Worksheets
By convention, scientists place the chemicals involved in a reaction into two basic categories: Products are substances that are produced in the reaction. Reactants and products are the two major components of a chemical reaction. Reactants are substances that start a chemical reaction. When a candle burns, the reactants are fuel (the candlewick and wax).
Reactants vs Products Difference and Comparison
Reactants are substances that start a chemical reaction. By convention, scientists place the chemicals involved in a reaction into two basic categories: Reactants and products are the two major components of a chemical reaction. Reactants are the starting material of a chemical reaction. This helps to explain what is happening during a reaction,.
Difference between Reactants and Products Difference Betweenz
Reactants are substances that start a chemical reaction. This helps to explain what is happening during a reaction,. Reactants and products are the two major components of a chemical reaction. The primary difference between reactants and products is that reactants are consumed during the reaction, whereas products are produced as a result of the reaction. When a candle burns, the.
Reactants vs Products Difference and Comparison
By convention, scientists place the chemicals involved in a reaction into two basic categories: Reactants and products are the two major components of a chemical reaction. This helps to explain what is happening during a reaction,. Reactants are substances that start a chemical reaction. Products are substances that are produced in the reaction.
(PPT) Objectives Differentiate between reactants and products in a
Products are the chemical species. By convention, scientists place the chemicals involved in a reaction into two basic categories: When a candle burns, the reactants are fuel (the candlewick and wax). Reactants are substances that start a chemical reaction. Reactants are the starting material of a chemical reaction.
Reactants vs Products Difference and Comparison
Reactants are the starting material of a chemical reaction. Reactants and products are the two major components of a chemical reaction. Products are substances that are produced in the reaction. When a candle burns, the reactants are fuel (the candlewick and wax). Products are the chemical species.
Difference Between Reactants and Products Definition, Properties
Reactants are substances that start a chemical reaction. Products are the chemical species. Reactants are the starting material of a chemical reaction. By convention, scientists place the chemicals involved in a reaction into two basic categories: Reactants and products are the two major components of a chemical reaction.
Difference Between Reactants and Products Definition, Properties
This helps to explain what is happening during a reaction,. The primary difference between reactants and products is that reactants are consumed during the reaction, whereas products are produced as a result of the reaction. Reactants are the starting material of a chemical reaction. Reactants are substances that start a chemical reaction. Reactants and products are the two major components.
Reactants Are Substances That Start A Chemical Reaction.
When a candle burns, the reactants are fuel (the candlewick and wax). Products are substances that are produced in the reaction. Products are the chemical species. Reactants and products are the two major components of a chemical reaction.
The Primary Difference Between Reactants And Products Is That Reactants Are Consumed During The Reaction, Whereas Products Are Produced As A Result Of The Reaction.
This helps to explain what is happening during a reaction,. By convention, scientists place the chemicals involved in a reaction into two basic categories: Reactants are the starting material of a chemical reaction.