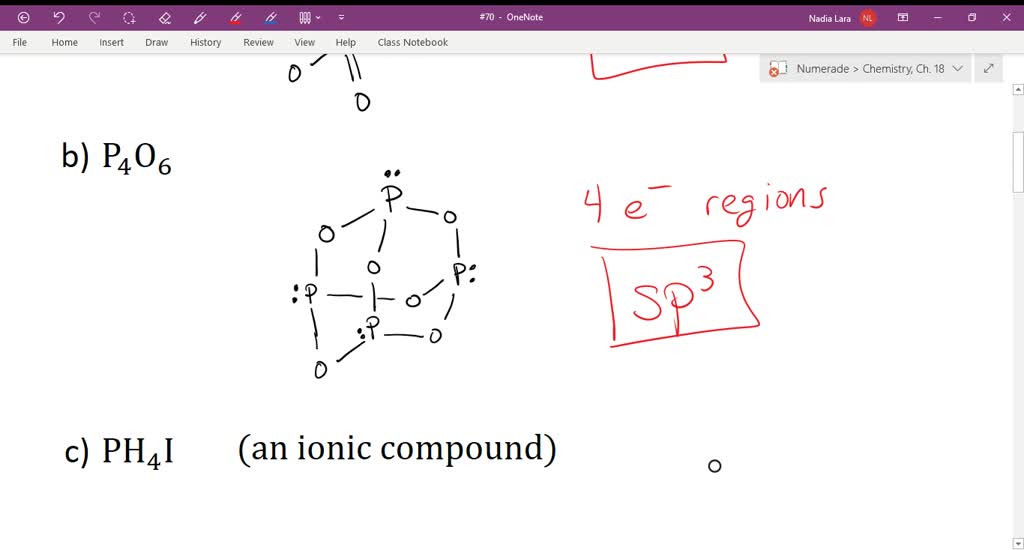
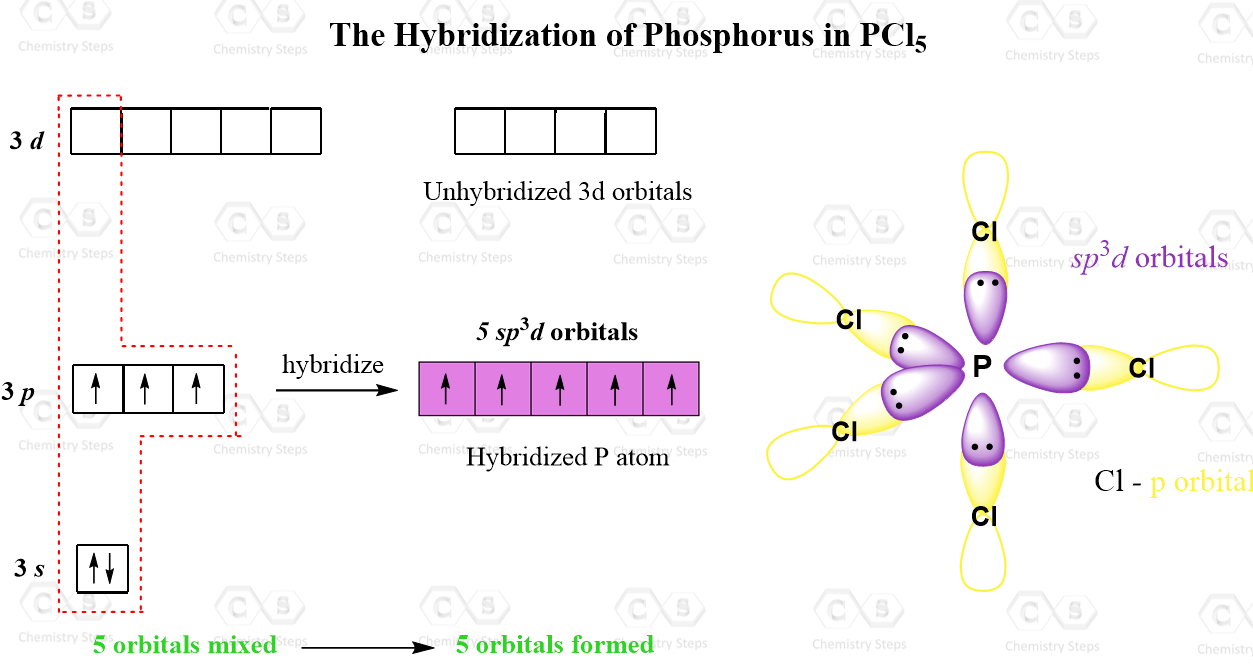
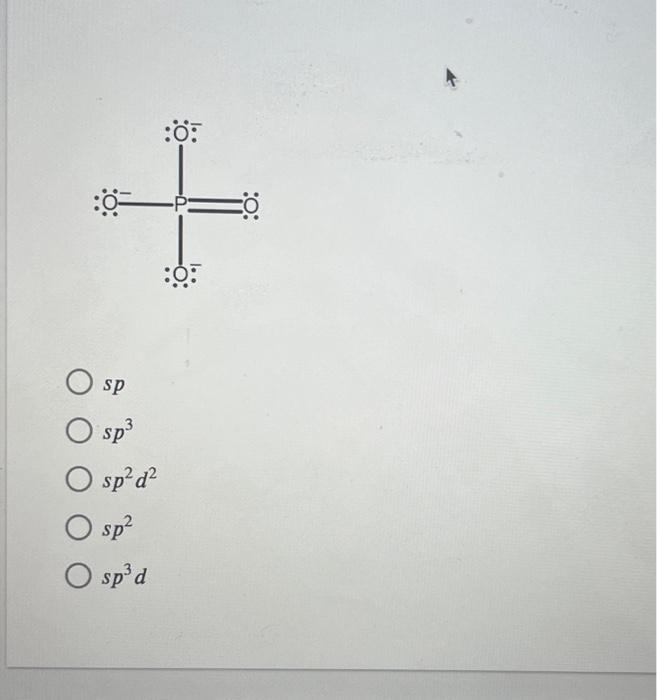
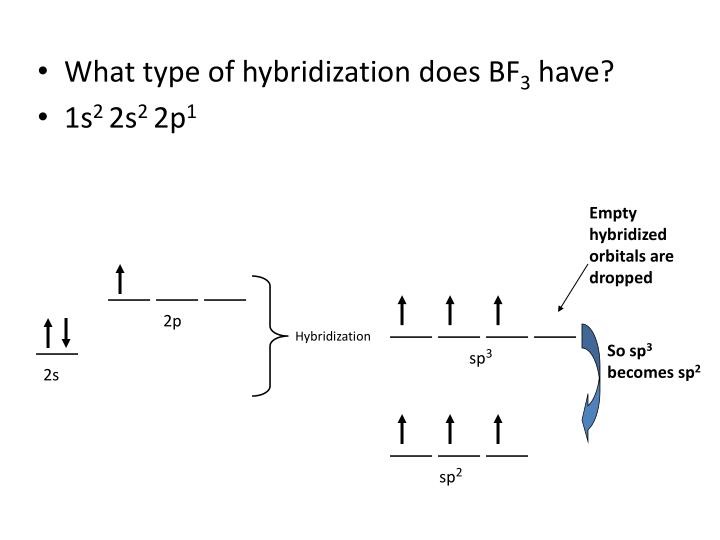
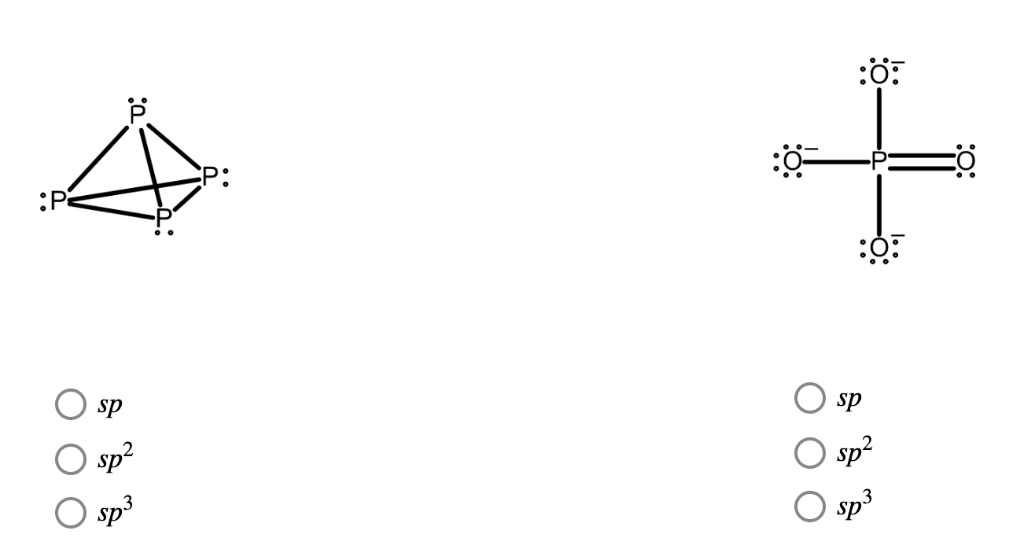What Is The Hybridization Of Phosphorus In Pcl3 - In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. The hybridization of p cl3 is sp3. What is the hybridization of phosphorus trichloride? To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p ocl3, p atom has.
In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. The hybridization of p cl3 is sp3. In p ocl3, p atom has. To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. What is the hybridization of phosphorus trichloride?
To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p ocl3, p atom has. The hybridization of p cl3 is sp3. What is the hybridization of phosphorus trichloride? In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons.
SOLVEDDescribe the hybridization of phosphorus in each of the
In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. In p ocl3, p atom has. The hybridization of p cl3 is sp3. What is the hybridization of phosphorus trichloride? To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its.
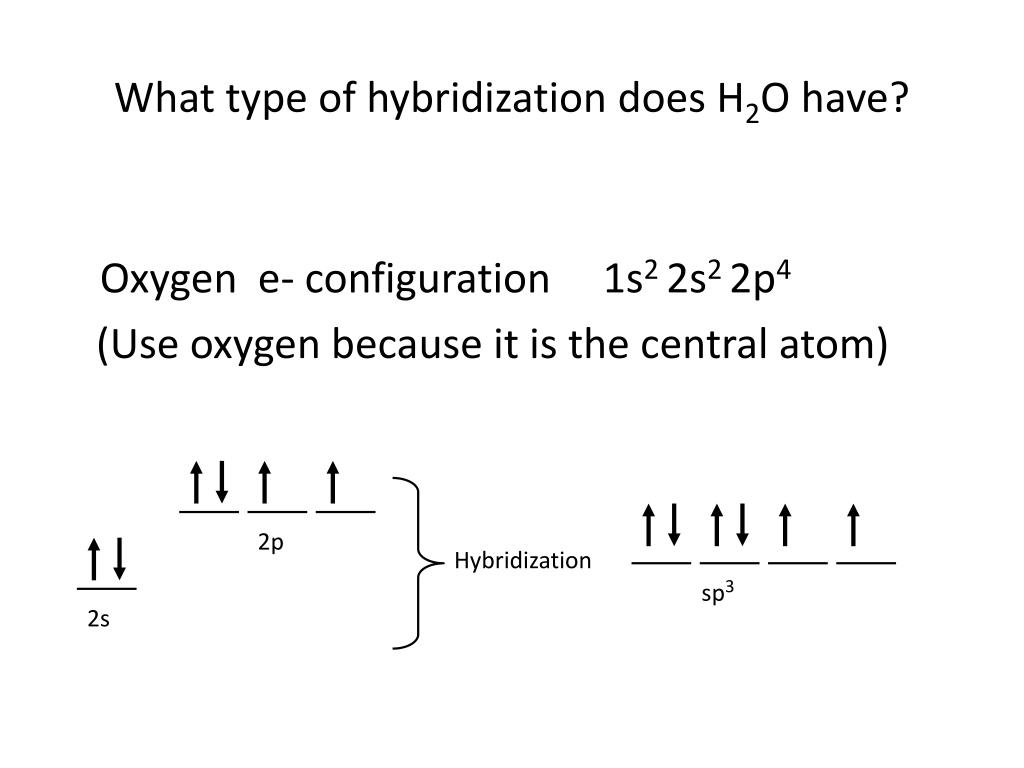
PPT Hybridization PowerPoint Presentation, free download ID2109983
What is the hybridization of phosphorus trichloride? To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p ocl3, p atom has. In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. The hybridization of p cl3 is sp3.
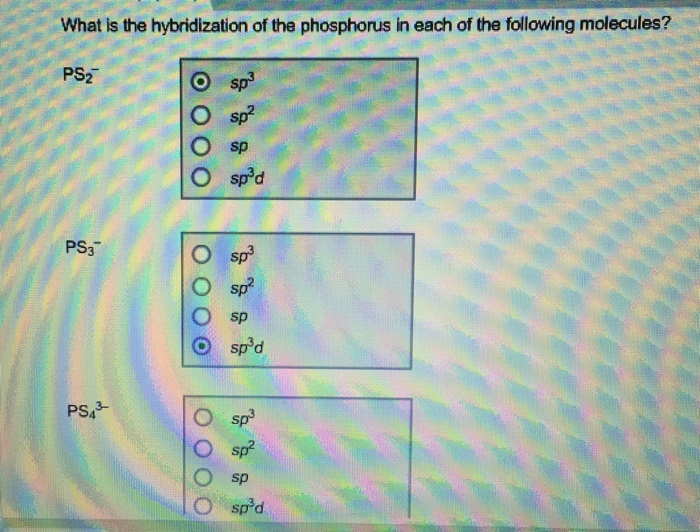
Solved What Is The Hybridization Of The Phosphorus N Each...
To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. What is the hybridization of phosphorus trichloride? In p ocl3, p atom has. The hybridization of p cl3 is sp3.
Phosphorus trichloride PCl₃ Molecular Geometry Hybridization
The hybridization of p cl3 is sp3. To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p ocl3, p atom has. What is the hybridization of phosphorus trichloride? In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons.
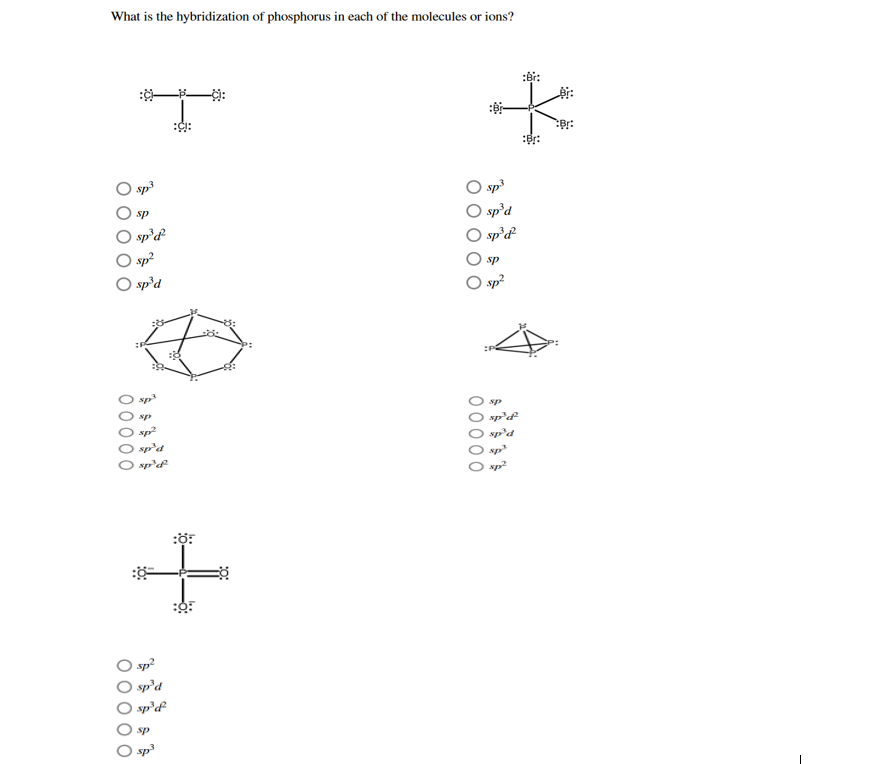
Solved What is the hybridization of phosphorus in each of
To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. The hybridization of p cl3 is sp3. In p ocl3, p atom has. What is the hybridization of phosphorus trichloride? In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons.
Hybridization Chart
What is the hybridization of phosphorus trichloride? In p ocl3, p atom has. In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. The hybridization of p cl3 is sp3. To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its.
Solved What is the hybridization of phosphorus in each of
In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. What is the hybridization of phosphorus trichloride? The hybridization of p cl3 is sp3. To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p ocl3, p atom has.
Solved Determine the hybridization of phosphorus in each
What is the hybridization of phosphorus trichloride? In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. The hybridization of p cl3 is sp3. To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p ocl3, p atom has.
PPT Hybridization PowerPoint Presentation ID2109983
The hybridization of p cl3 is sp3. What is the hybridization of phosphorus trichloride? To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p ocl3, p atom has. In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons.
Solved What is the hybridization of phosphorus in each of
The hybridization of p cl3 is sp3. In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. In p ocl3, p atom has. What is the hybridization of phosphorus trichloride? To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its.
What Is The Hybridization Of Phosphorus Trichloride?
The hybridization of p cl3 is sp3. In p cl3, p atom has 3 bond pairs of electrons and one lone pair of electrons. To understand the hybridization scheme for pcl3 (phosphorus trichloride), we need to look at its. In p ocl3, p atom has.