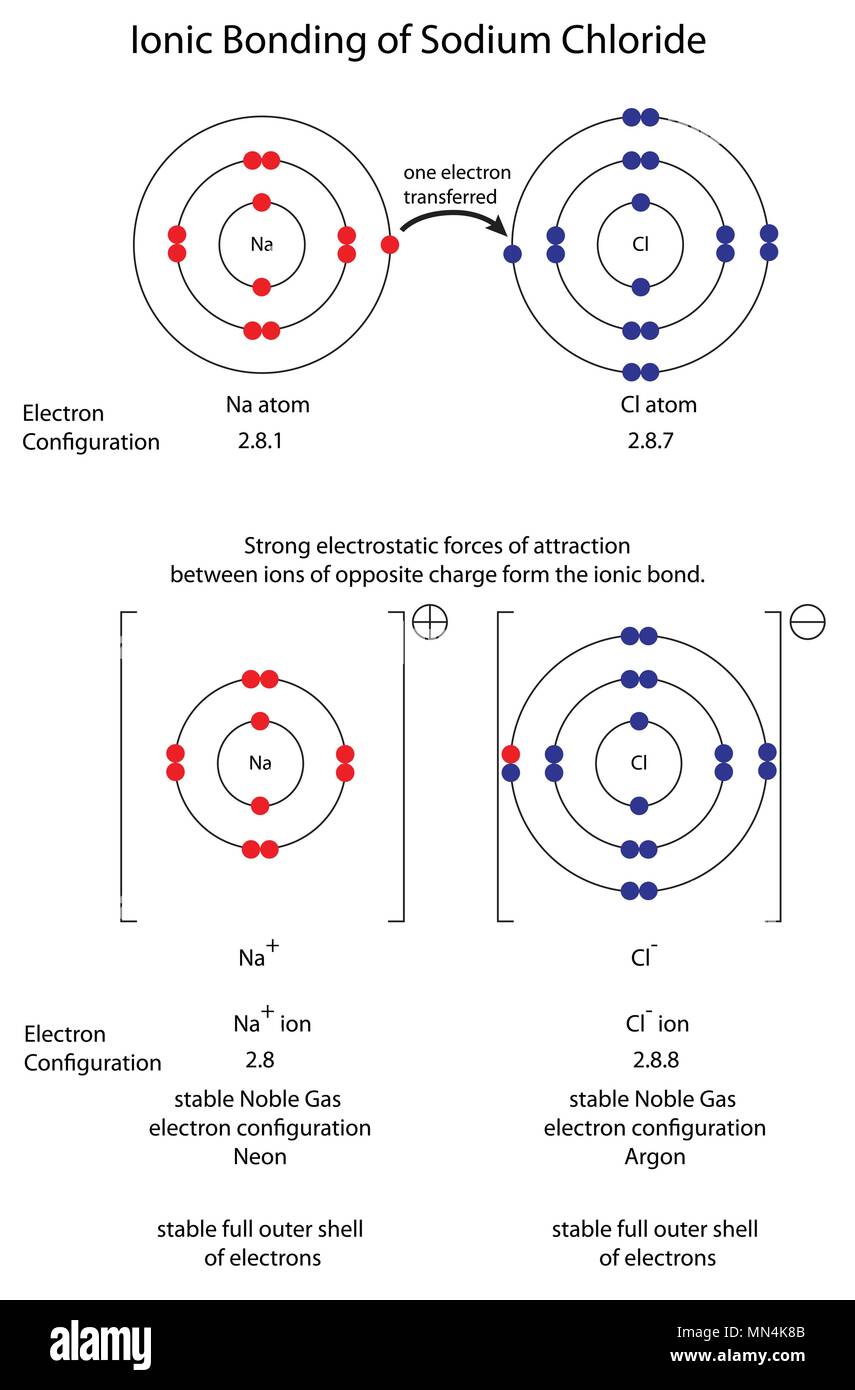
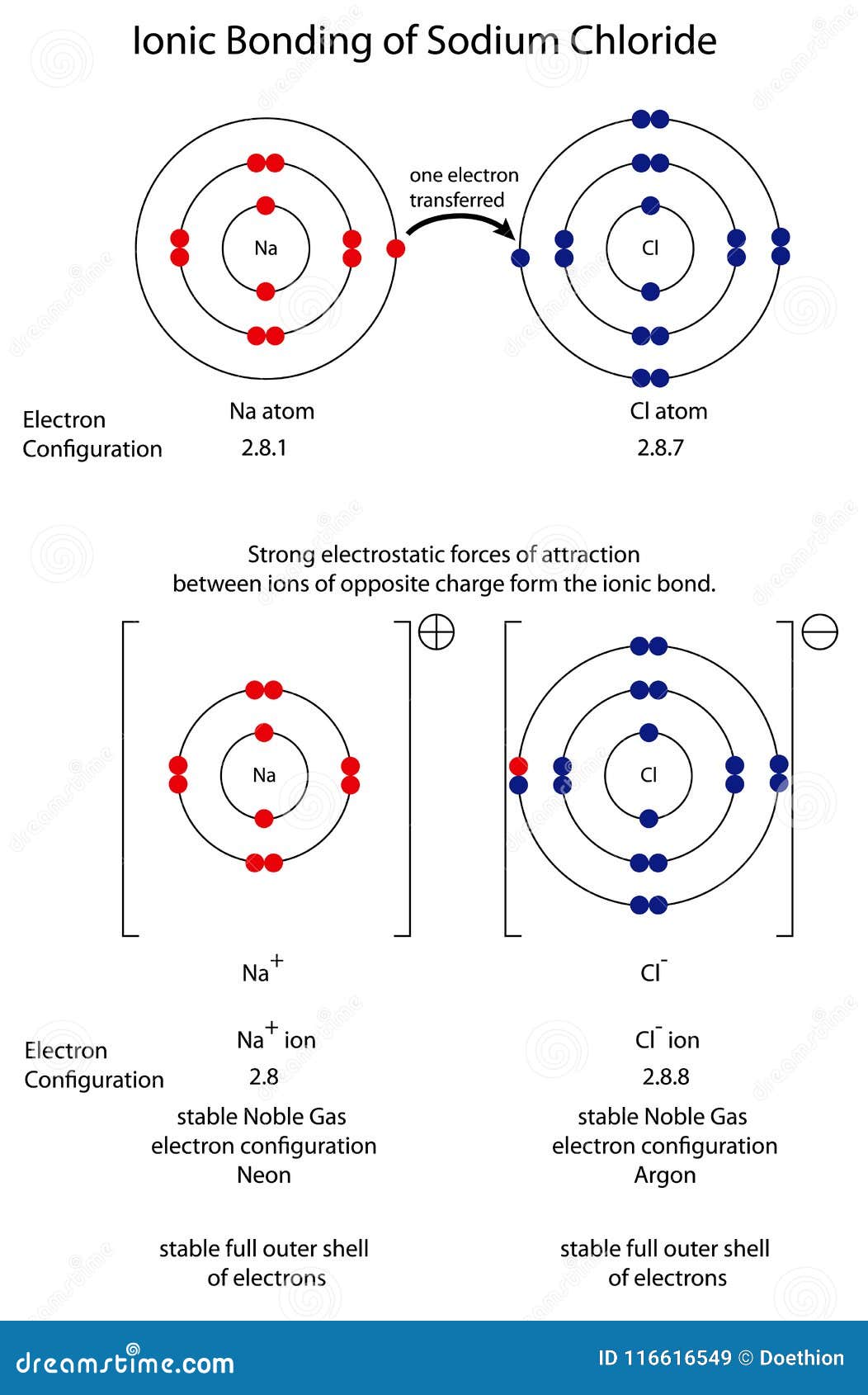
The Ionic Bond Of Sodium Chloride Is Formed When - The ions are held together in the lattice by strong ionic bonds between the oppositely charged. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of.
The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of. The ions are held together in the lattice by strong ionic bonds between the oppositely charged. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus.
The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The ions are held together in the lattice by strong ionic bonds between the oppositely charged.
ionic bond Definition, Properties, Examples, & Facts Britannica
The ions are held together in the lattice by strong ionic bonds between the oppositely charged. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of.
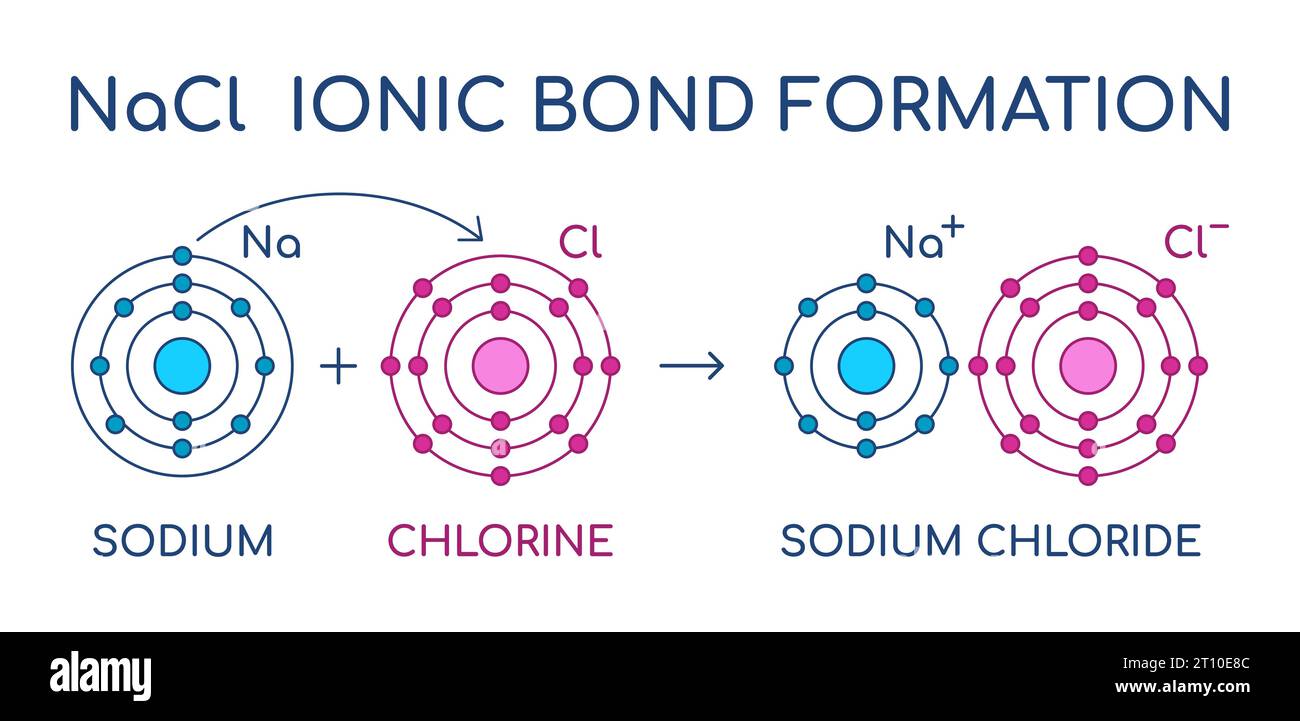
Sodium Chloride ionic bond formation. NaCl structure. Sodium and
So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of. The ions are held together in the lattice by strong ionic bonds between the oppositely charged.
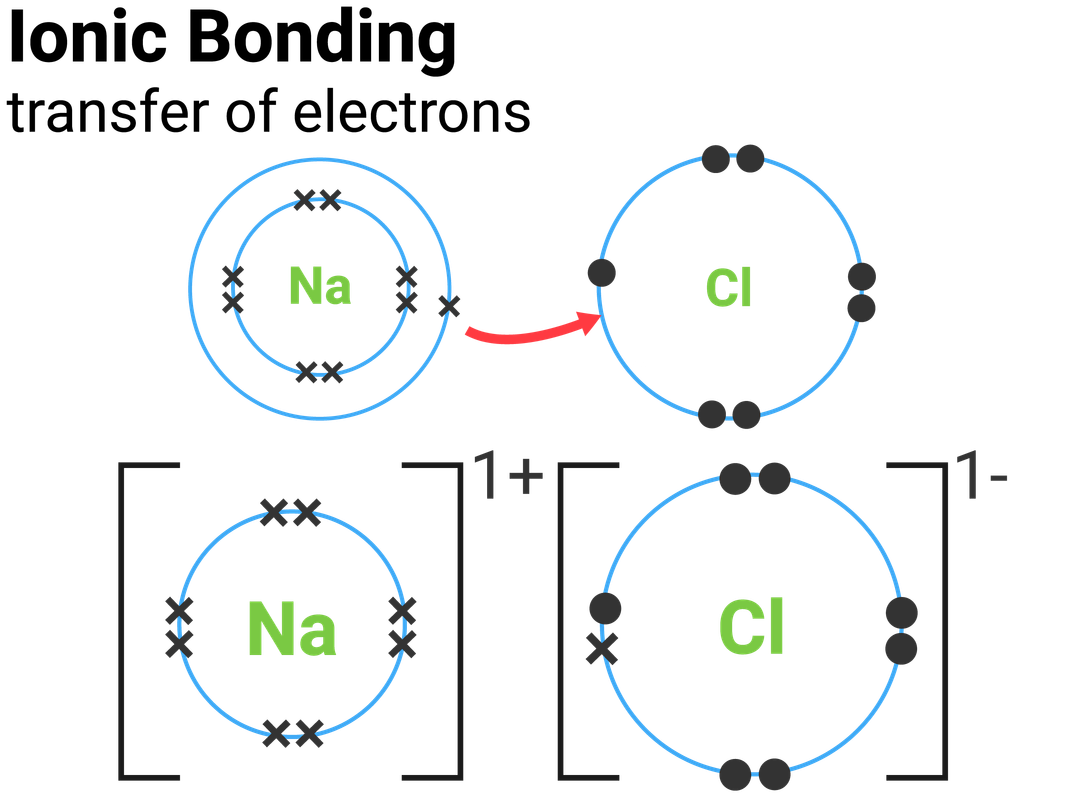
Sodium Chloride Ionic Bonding Diagram
The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of. The ions are held together in the lattice by strong ionic bonds between the oppositely charged. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus.
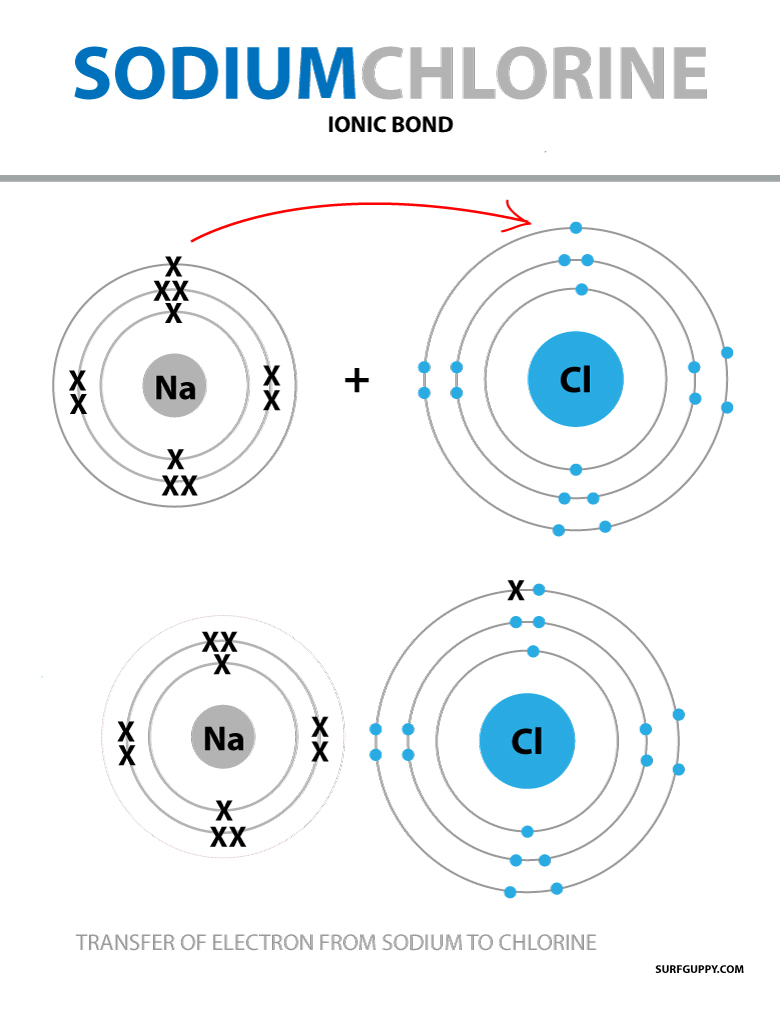
What is Ionic Bond Surfguppy Chemistry made easy visual learning
The ions are held together in the lattice by strong ionic bonds between the oppositely charged. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of.
Ionic Bond in Sodium Chloride Stock Vector Illustration of sodium
The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of. The ions are held together in the lattice by strong ionic bonds between the oppositely charged. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus.
Diagram Of Ionic Bonding In Sodium Chloride
So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The ions are held together in the lattice by strong ionic bonds between the oppositely charged. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of.
Diagram To Show Ionic Bonding in Sodium Chloride Stock Illustration
The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The ions are held together in the lattice by strong ionic bonds between the oppositely charged.
Ionic bond Definition, Properties, Examples, & Facts Britannica
The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The ions are held together in the lattice by strong ionic bonds between the oppositely charged.
What Is An Ionic Bond Sciencing Ionic Bonding Ionic Chemical Bond
The ions are held together in the lattice by strong ionic bonds between the oppositely charged. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of.
Diagram to show ionic bonding in sodium chloride Stock Vector Image
The ions are held together in the lattice by strong ionic bonds between the oppositely charged. So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of.
The Ions Are Held Together In The Lattice By Strong Ionic Bonds Between The Oppositely Charged.
So, the excess one electron is transferred to a chlorine atom from n a(sodium) atom and thus. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of.




.PNG)