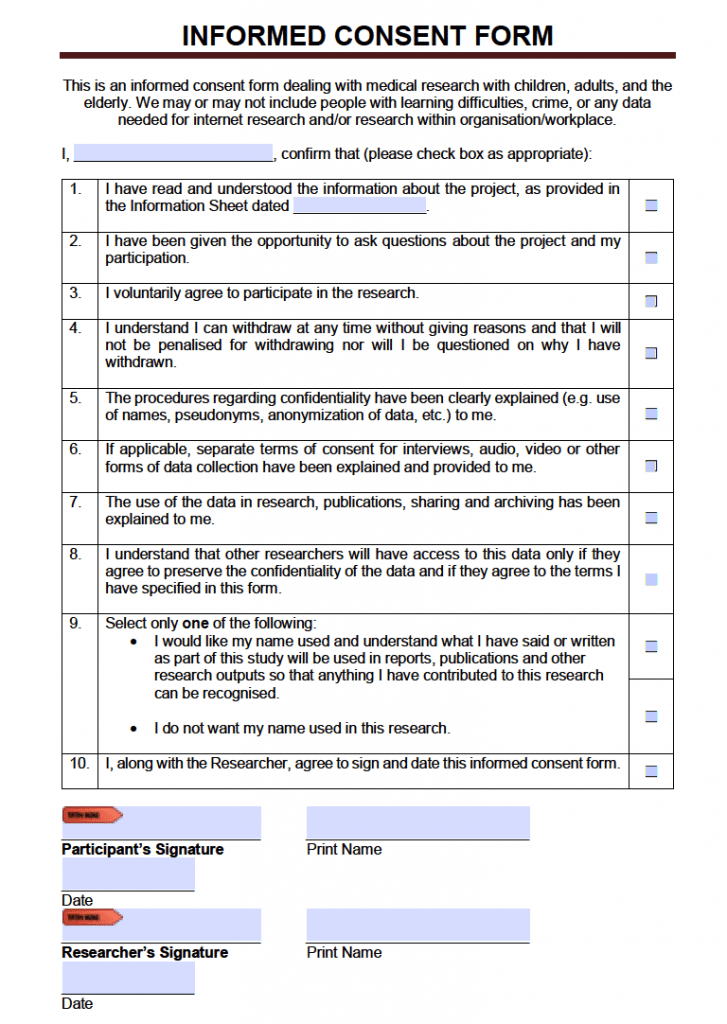
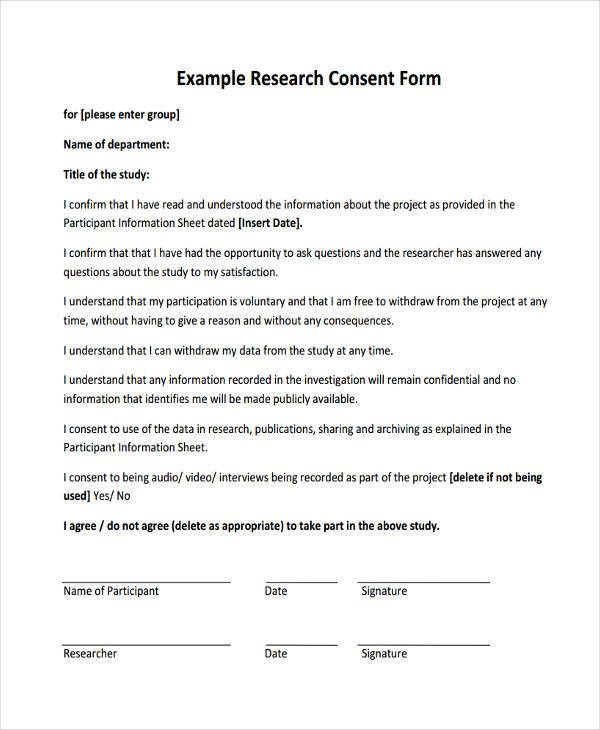
Example Of A Consent Form For Research Study - Participants must have (describe inclusion. • you are being asked to be in a research study of [insert general statement about study]. • you were selected as a possible participant because [. Template for creating an informed consent form this template is for research projects that use questionnaires/surveys, interviews, focus group. Customize this template to reflect the specifics of your study and. This section details what will be involved in your research study from a participant’s point of view, and in the order they will experience it. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. We estimate that 20 participants who (describe population) will enroll in this study. Avoid common problems with consent forms. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
We estimate that 20 participants who (describe population) will enroll in this study. When completing and irb submission in irbis, please fill in the. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. Avoid common problems with consent forms. Participants must have (describe inclusion. This section details what will be involved in your research study from a participant’s point of view, and in the order they will experience it. Template for creating an informed consent form this template is for research projects that use questionnaires/surveys, interviews, focus group. Customize this template to reflect the specifics of your study and. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. • you were selected as a possible participant because [.
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. We estimate that 20 participants who (describe population) will enroll in this study. • you are being asked to be in a research study of [insert general statement about study]. When completing and irb submission in irbis, please fill in the. This section details what will be involved in your research study from a participant’s point of view, and in the order they will experience it. Participants must have (describe inclusion. Avoid common problems with consent forms. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. Template for creating an informed consent form this template is for research projects that use questionnaires/surveys, interviews, focus group. • you were selected as a possible participant because [.
ND example consent UNIVERSITY OF NOTRE DAME INFORMED CONSENT
When completing and irb submission in irbis, please fill in the. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. • you were selected as a possible participant because [..
Ssurvivor Consent Form For Research Interview
We estimate that 20 participants who (describe population) will enroll in this study. • you are being asked to be in a research study of [insert general statement about study]. • you were selected as a possible participant because [. When completing and irb submission in irbis, please fill in the. Template for creating an informed consent form this template.
Free Informed Consent Form for Research Example PDF Word
• you are being asked to be in a research study of [insert general statement about study]. Avoid common problems with consent forms. We estimate that 20 participants who (describe population) will enroll in this study. Template for creating an informed consent form this template is for research projects that use questionnaires/surveys, interviews, focus group. The following templates and samples.
Study Consent Form Template DocTemplates
We estimate that 20 participants who (describe population) will enroll in this study. • you were selected as a possible participant because [. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. These consent form templates have been posted for your reference. Participants must have (describe inclusion.
Research Consent Form Sample Tufts University Free Download
The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. • you were selected as a possible participant because [. When completing and irb submission in irbis, please fill in the..
Research Consent Form Fill Out, Sign Online and Download PDF
This section details what will be involved in your research study from a participant’s point of view, and in the order they will experience it. These consent form templates have been posted for your reference. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. Avoid common problems with consent.
Example Of A Consent Form For Research Study Study Poster
• you are being asked to be in a research study of [insert general statement about study]. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Customize this template to reflect the specifics of your study and. Participants must have (describe inclusion. • you were selected as a possible participant.
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
Avoid common problems with consent forms. These consent form templates have been posted for your reference. Template for creating an informed consent form this template is for research projects that use questionnaires/surveys, interviews, focus group. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. When completing and irb submission in.
Informed Consent Form Template for clinical trials
This section details what will be involved in your research study from a participant’s point of view, and in the order they will experience it. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. Customize this template to reflect the specifics of your study and. • you are being.
9+ Research Consent Form Templates Sample Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. • you are being asked to be in a research study of [insert general statement about study]. Participants must have (describe.
These Consent Form Templates Have Been Posted For Your Reference.
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Template for creating an informed consent form this template is for research projects that use questionnaires/surveys, interviews, focus group. This section details what will be involved in your research study from a participant’s point of view, and in the order they will experience it. Customize this template to reflect the specifics of your study and.
Avoid Common Problems With Consent Forms.
Participants must have (describe inclusion. The following templates and samples are provided for investigators who are designing consent, assent, or permission forms for research with. • you were selected as a possible participant because [. We estimate that 20 participants who (describe population) will enroll in this study.
When Completing And Irb Submission In Irbis, Please Fill In The.
• you are being asked to be in a research study of [insert general statement about study].