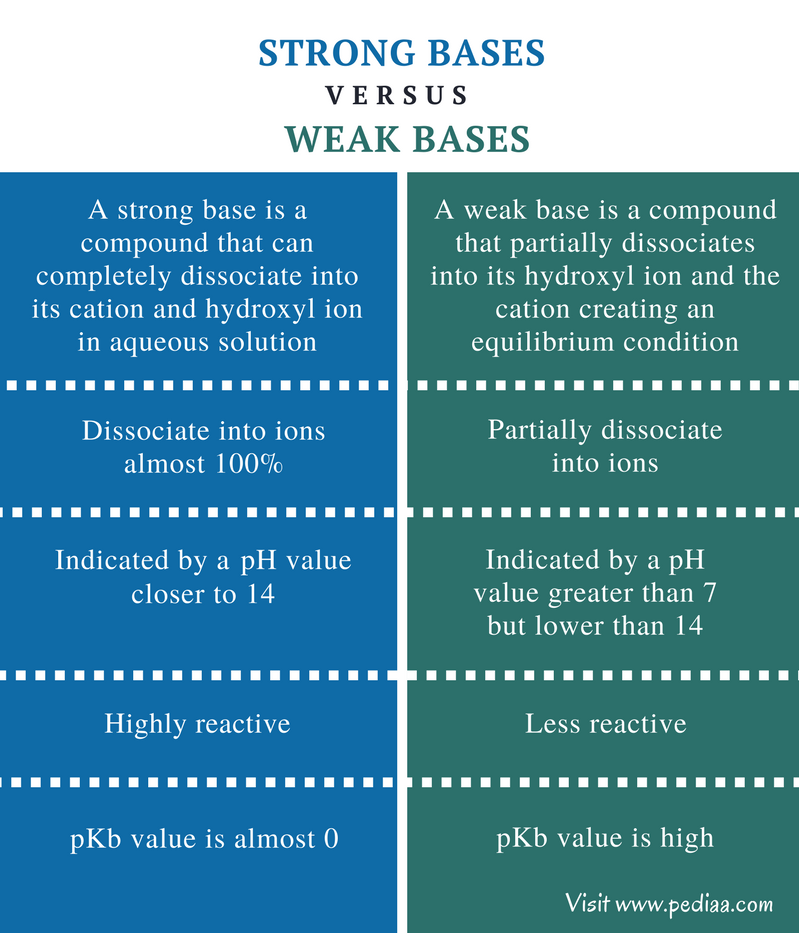
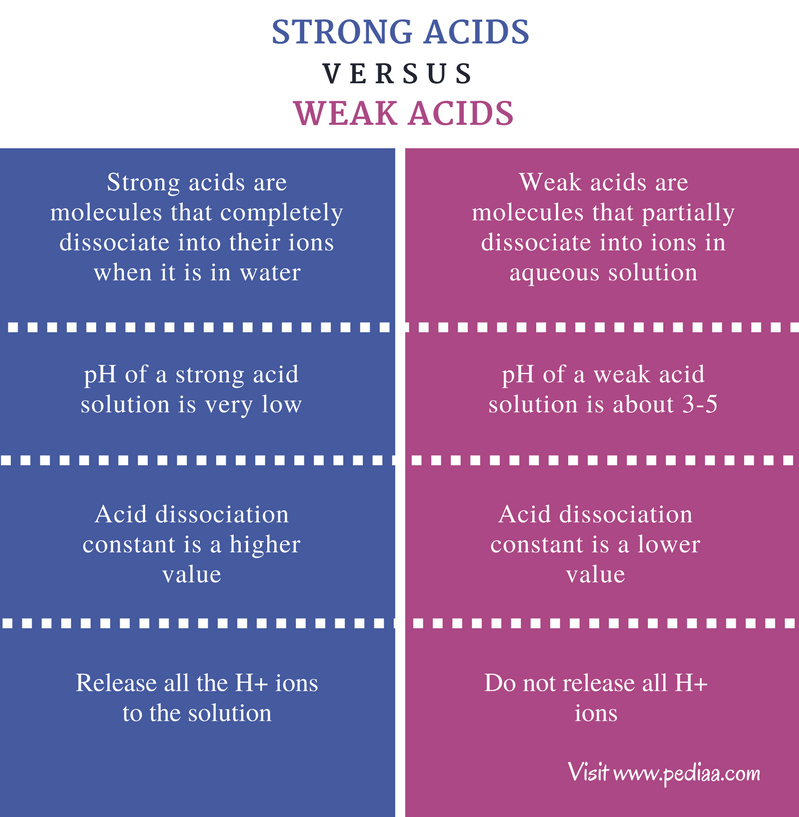
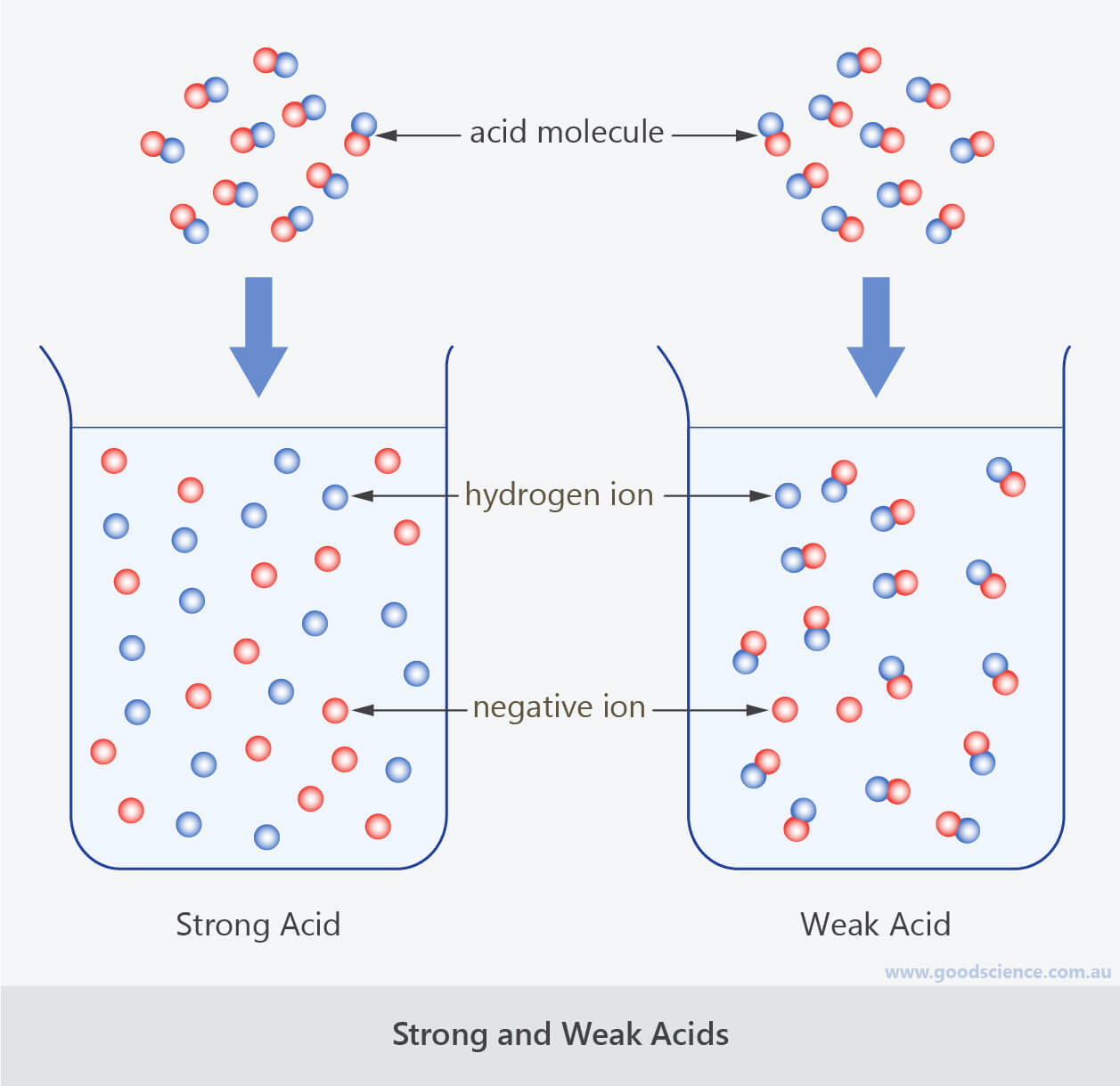
Differentiate Between Strong Acid And Weak Acid - Define a strong and a weak acid and base. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Determine if a salt produces an acidic or a. Recognize an acid or a base as strong or weak. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially.
Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Recognize an acid or a base as strong or weak. Define a strong and a weak acid and base. Determine if a salt produces an acidic or a. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water.
Determine if a salt produces an acidic or a. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Recognize an acid or a base as strong or weak. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Define a strong and a weak acid and base.
Difference Between Strong and Weak Bases Definition, Properties
Define a strong and a weak acid and base. Recognize an acid or a base as strong or weak. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Determine if a salt produces an acidic or a. Strong acid is an acid that completely dissociates in an aqueous solution,.
Strong Acid and Weak Acid
Define a strong and a weak acid and base. Determine if a salt produces an acidic or a. Recognize an acid or a base as strong or weak. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Strong acids and weak acids are both types of acids, but they.
[Solved] How can a chemist differentiate between a strong acid and weak
Determine if a salt produces an acidic or a. Define a strong and a weak acid and base. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Recognize an acid or a base as strong or weak. Strong acids and weak acids are both types of acids, but they.
Difference Between Strong and Weak Acids Definition, Properties, Examples
Define a strong and a weak acid and base. Determine if a salt produces an acidic or a. Recognize an acid or a base as strong or weak. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Strong acid is an acid that completely dissociates in an aqueous solution,.
Difference between Strong and Weak Acid Difference Betweenz
Recognize an acid or a base as strong or weak. Define a strong and a weak acid and base. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Determine.
Strong Acid vs Weak Acid Difference and Comparison
Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Determine if a salt produces an acidic or a. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Recognize an acid or a base as strong or weak. Define.
Strong Weak Acid Base Chart A Visual Reference of Charts Chart Master
Determine if a salt produces an acidic or a. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Define a strong and a weak acid and base. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Recognize an.
Strong Acid and Weak Acid CorbinkruwAllen
Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Determine if a salt produces an acidic or a. Recognize an acid or a base as strong or weak. Define a strong and a weak acid and base. Strong acid is an acid that completely dissociates in an aqueous solution,.
[Solved] 1. explain and differentiate between a strong entities and a
Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Determine if a salt produces an acidic or a. Define a strong and a weak acid and base. Recognize an.
Difference Between Weak and Strong Acid Compare the Difference
Define a strong and a weak acid and base. Strong acid is an acid that completely dissociates in an aqueous solution, while weak acid is an acid that partially. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Determine if a salt produces an acidic or a. Recognize an.
Strong Acid Is An Acid That Completely Dissociates In An Aqueous Solution, While Weak Acid Is An Acid That Partially.
Recognize an acid or a base as strong or weak. Define a strong and a weak acid and base. Strong acids and weak acids are both types of acids, but they differ in their ability to dissociate in water. Determine if a salt produces an acidic or a.