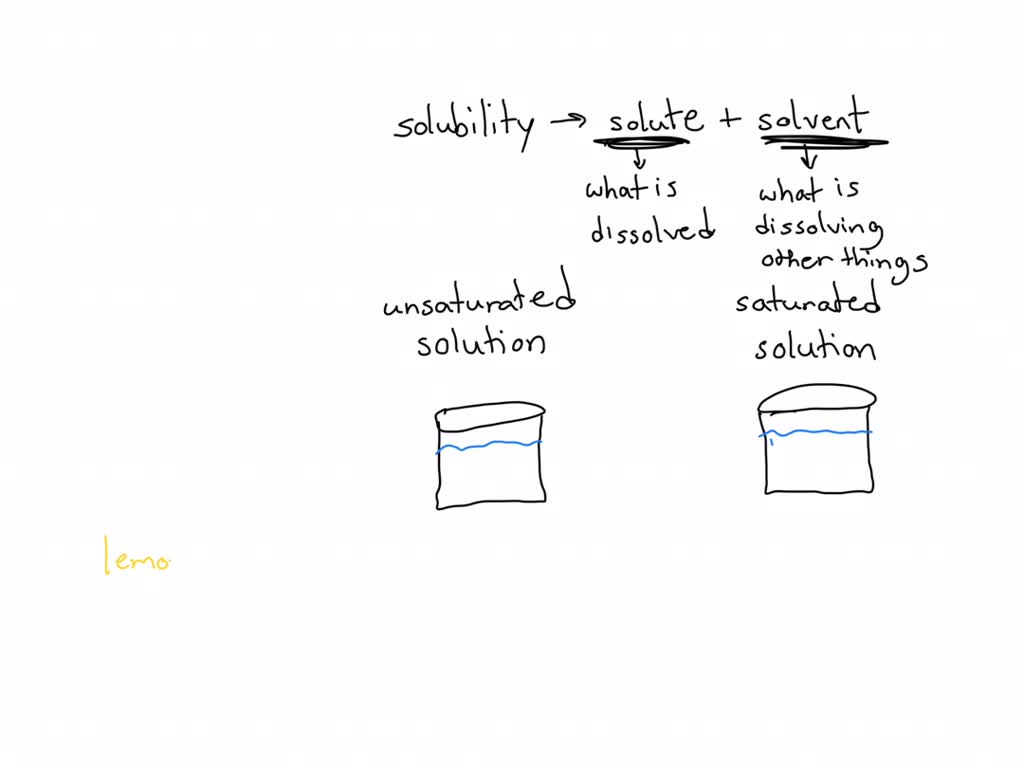
Differentiate Between Saturated And Unsaturated Solutions - (a) differentiate between a saturated and an unsaturated solution. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. An unsaturated solution is a solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. How will you test whether a given solution is saturated or not? A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. An unsaturated solution is a solution.
An unsaturated solution is a solution. How will you test whether a given solution is saturated or not? A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. An unsaturated solution is a solution. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. (a) differentiate between a saturated and an unsaturated solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types:
(a) differentiate between a saturated and an unsaturated solution. How will you test whether a given solution is saturated or not? The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: An unsaturated solution is a solution.
SOLVED Explain the principle of solubility in your own words, and
A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. How will you test whether a given solution is saturated or not? (a) differentiate between a saturated and an unsaturated solution. An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is.
Differentiate between saturated and unsaturated hydrocarbons CBSE
Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. (a) differentiate between a.
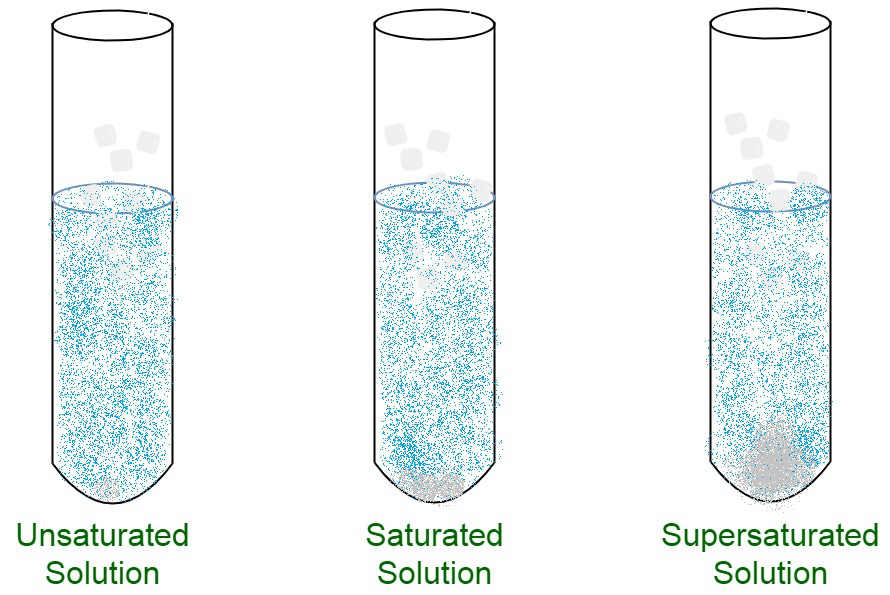
Saturated Unsaturated Supersaturated Images
An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. (a) differentiate between a saturated and an unsaturated solution. An unsaturated solution is a solution.
SOLVED Explain the principle of solubility in your own words, and
A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. (a) differentiate between a saturated and an unsaturated solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. An unsaturated solution is a solution. An unsaturated solution is a solution.
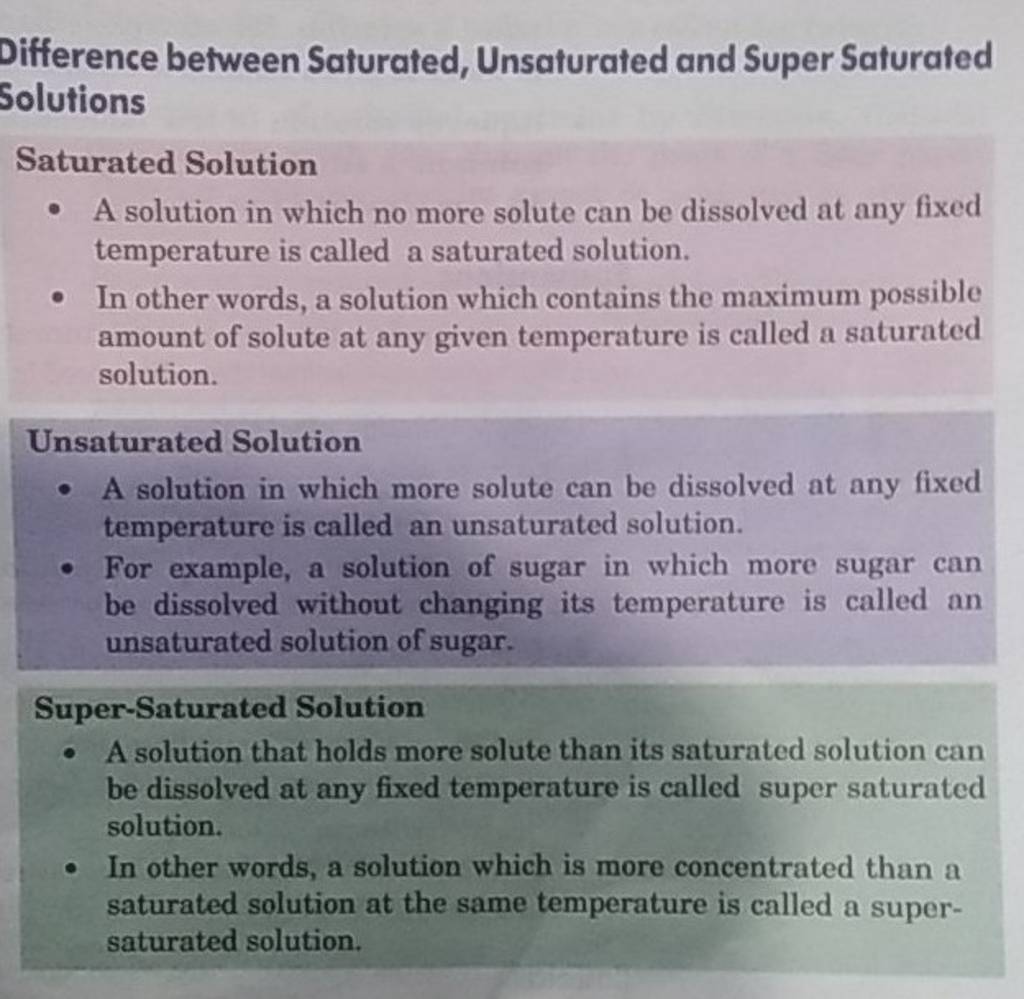
Difference between Saturated, Unsaturated and Super Saturated Solutions S..
A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. An unsaturated solution is a solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: The main difference between saturated and unsaturated solutions is that a saturated solution is a solution.
3. Differentiate between saturated and unsaturated ounds? 4. Write the el..
(a) differentiate between a saturated and an unsaturated solution. An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: The main difference between saturated and unsaturated.
Solutions How can one differentiate between saturated unsaturated
Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: How will you test whether a given solution is saturated or not? The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. A saturated solution is a solution that contains the maximum.
Saturated and Unsaturated Solutions
(a) differentiate between a saturated and an unsaturated solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. An unsaturated solution is a solution. How will you test whether a given.
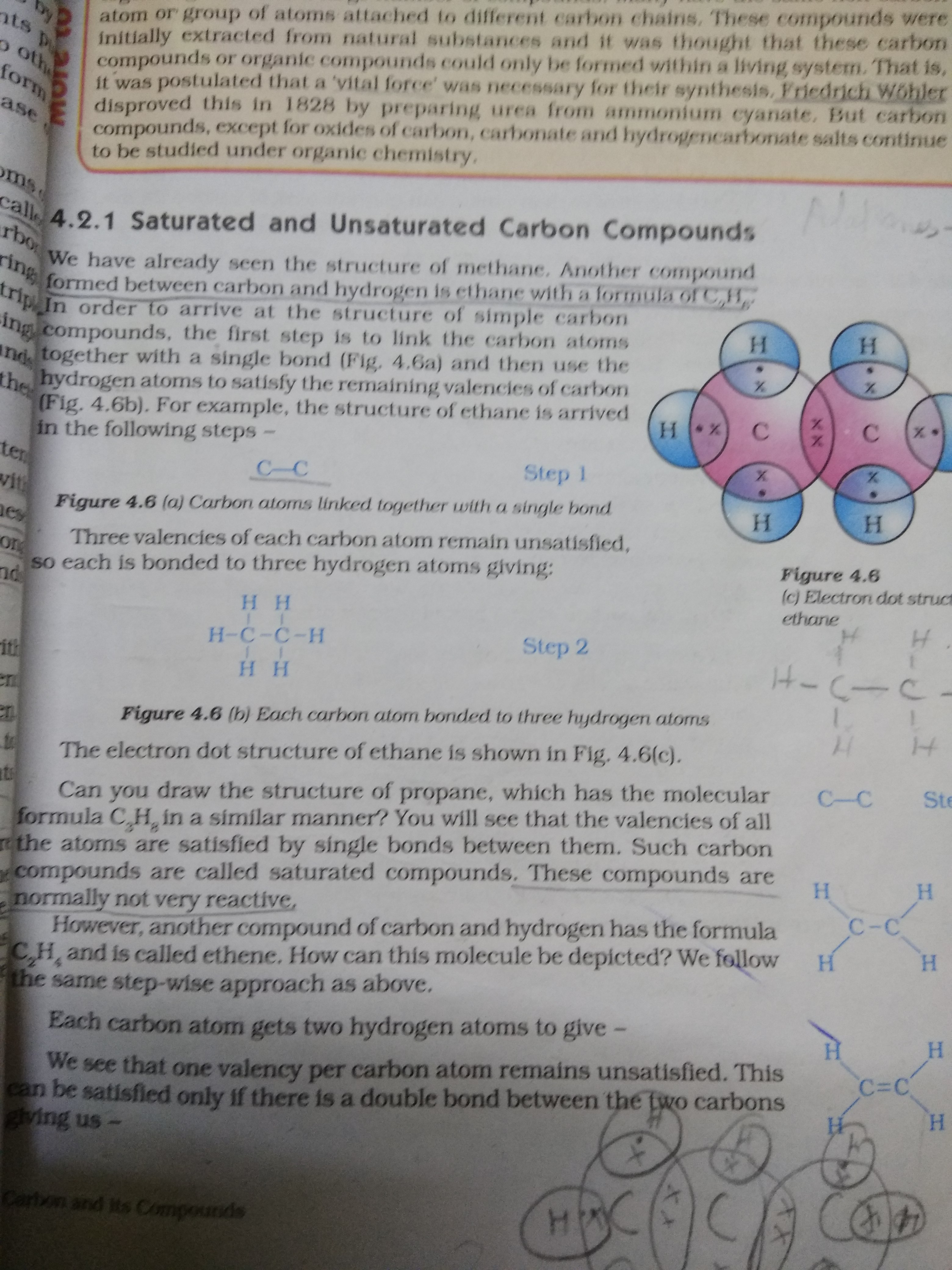
Difference Between Saturated and Unsaturated Hydrocarbons Definition
How will you test whether a given solution is saturated or not? An unsaturated solution is a solution. (a) differentiate between a saturated and an unsaturated solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. The main difference between saturated and unsaturated solutions is that a saturated solution is a.
Difference Between Saturated and Unsaturated Compounds Definition
The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. How will you test.
Depending On The Amount Of Solute Dissolved In A Given Amount Of Solvent, Solutions Can Be Classified Into Three Types:
How will you test whether a given solution is saturated or not? (a) differentiate between a saturated and an unsaturated solution. An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving.
A Saturated Solution Is A Solution That Contains The Maximum Amount Of Solute That Is Capable Of Dissolving.
An unsaturated solution is a solution. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains.