Differentiate Between Octahedral Fe 2 And Fe 3 In Fe24O32 - Start with an oh energy. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex. I understand that fe(ii) has 6. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions.
Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Start with an oh energy. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex. I understand that fe(ii) has 6.
I understand that fe(ii) has 6. Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. Start with an oh energy. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites.
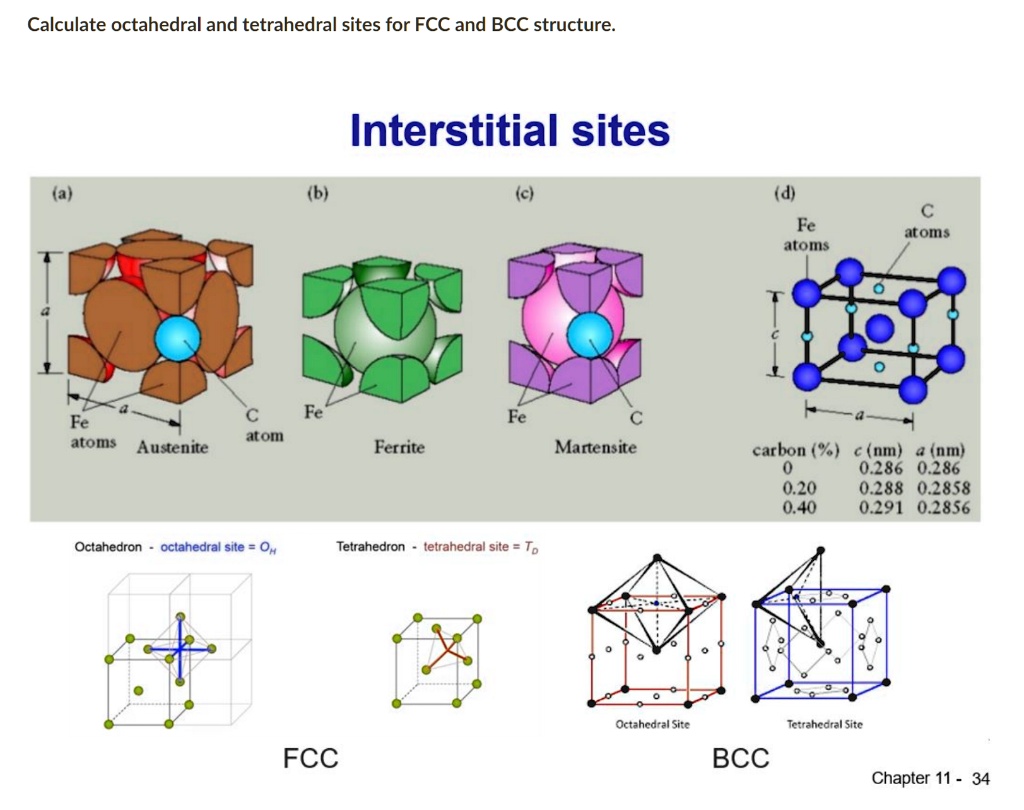
calculate octahedral and tetrahedral sites for fcc and bcc structure
This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. I understand that fe(ii) has 6. Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex..
Solved If an octahedral Fe*2 complex is which
This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Start with an oh energy. I understand that fe(ii) has 6. Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. Now combine the tm dao and ligand fo components to form the energy level diagram.
Distortions for a 3d 2 configuration yielding the transition from
Start with an oh energy. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex. I understand that fe(ii) has 6. Both fe 2+ and fe 3+ ions give octahedral cyano anionic.
Diagram Fe 2+/ Fe 2+ + Fe 3+ vs Mn/(Mn+Mg) showing comparison between
I understand that fe(ii) has 6. Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. Start with an oh energy. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Now combine the tm dao and ligand fo components to form the energy level diagram.
Fig S9 Schematic electronic DOS of Fe 3+ in octahedral O h and
Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. I understand that fe(ii) has 6. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex..
Plot of Li versus octahedral Fe 2þ cations for the magmatic grains. All
Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. I understand that fe(ii) has 6. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Start with an oh energy. Now combine the tm dao and ligand fo components to form the energy level diagram.
Why is Fe2+ more easily oxidized to Fe3+? Life Set Go
I understand that fe(ii) has 6. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex..
Octahedral (Fe tot þ Mn þ TiÀAl VI ) vs. octahedral (MgLi) diagram
Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. I understand that fe(ii) has 6. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Start with an oh energy. Now combine the tm dao and ligand fo components to form the energy level diagram.
Preparation and Comparative Evaluation of Fe+2/Fe+3 and Mg+2/Fe+3 LDHs
This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Start with an oh energy. I understand that fe(ii) has 6. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex. Both fe 2+ and fe 3+ ions give octahedral cyano anionic.
What is the standard reduction potential (E°) Fe3+ Fe ? Given that Fe2
I understand that fe(ii) has 6. Start with an oh energy. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. Now combine the tm dao and ligand fo components to form the energy level diagram for a c4v tm complex. Both fe 2+ and fe 3+ ions give octahedral cyano anionic.
Now Combine The Tm Dao And Ligand Fo Components To Form The Energy Level Diagram For A C4V Tm Complex.
Both fe 2+ and fe 3+ ions give octahedral cyano anionic complex ions with cyanide ions. This structure is called a spinel, with $\ce{fe^{2+}}$ ion in tetrahedral coordination and $\ce{fe^{3+}}$> ions in octahedral sites. I understand that fe(ii) has 6. Start with an oh energy.