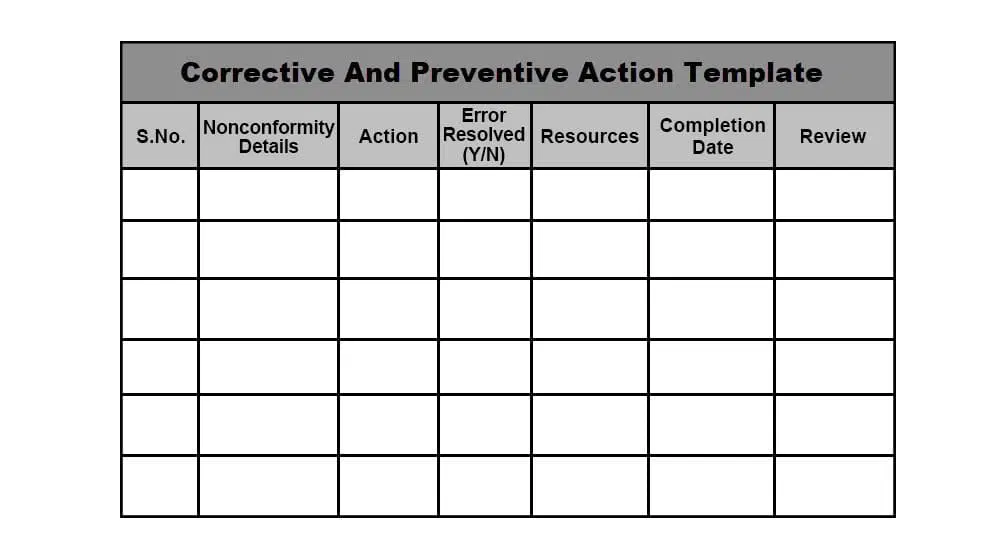
Corrective Action And Preventive Action Capa Process - Corrective and preventive actions (capa) inspectional objectives. Corrective action and preventive action documentation can demonstrate to fda that the. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
Corrective and preventive actions (capa) inspectional objectives. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective action and preventive action documentation can demonstrate to fda that the.
CAPA When to Take Corrective Action or Preventive Action?
In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives.
What Is Capa Corrective Action And Preventive Action Corrective
In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives.
Updated Guide to Corrective Action and Preventive Action (CAPA) for
In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives.
Mastering Corrective and Preventive Action (CAPA) Procedures for
Corrective action and preventive action documentation can demonstrate to fda that the. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions (capa) inspectional objectives.
CAPA Systems 5 Essential Elements CAPA Software Arena
Corrective and preventive actions (capa) inspectional objectives. Corrective action and preventive action documentation can demonstrate to fda that the. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
What is Corrective and Preventive Action (CAPA)? PM Study Circle
Corrective action and preventive action documentation can demonstrate to fda that the. In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions (capa) inspectional objectives.
Understanding Corrective and Preventive Action A Systematic Approach
Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
CAPA Corrective and preventive action. Vector stock illustration
Corrective action and preventive action documentation can demonstrate to fda that the. Corrective and preventive actions (capa) inspectional objectives. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
Corrective and Preventive Actions CAPA BioPharma Services
In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective and preventive actions (capa) inspectional objectives. Corrective action and preventive action documentation can demonstrate to fda that the.
Corrective and Preventive Action (CAPA) Process in GMP Industrial
Corrective and preventive actions (capa) inspectional objectives. Corrective action and preventive action documentation can demonstrate to fda that the. In this article, you will learn capa, ca (corrective action), pa (preventive action), the.
Corrective And Preventive Actions (Capa) Inspectional Objectives.
In this article, you will learn capa, ca (corrective action), pa (preventive action), the. Corrective action and preventive action documentation can demonstrate to fda that the.