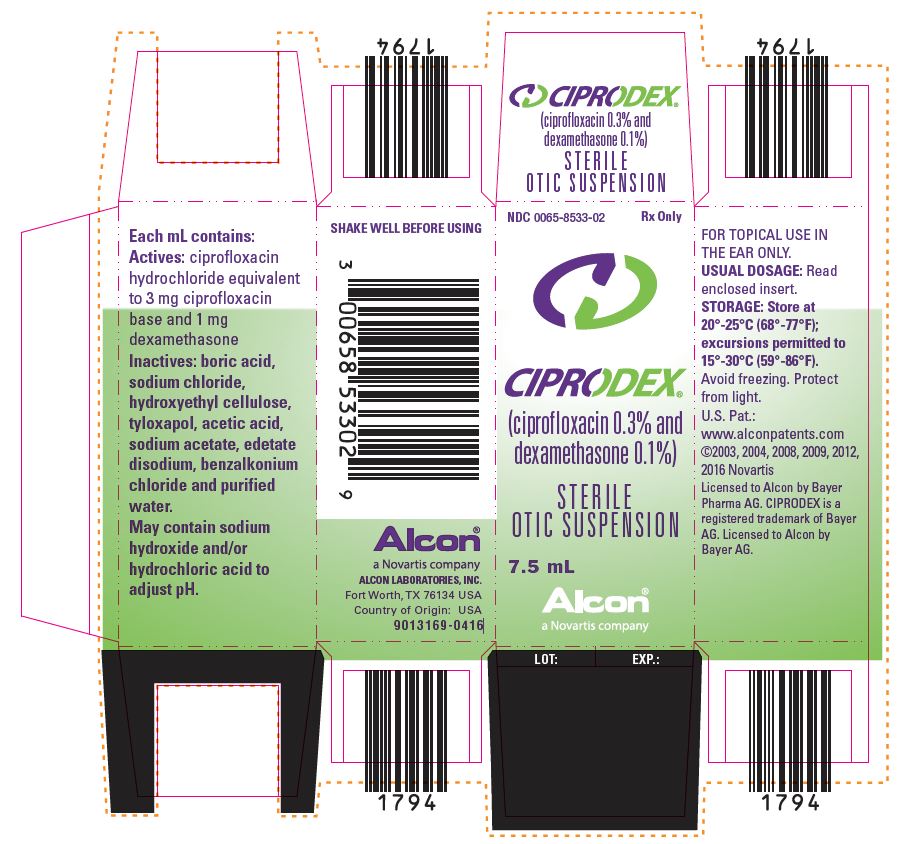
Ciprodex Diagnosis Code - 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. List of products in the national drug code with proprietary name ciprodex. Ciprodex® is dosed 7 days for all indications, while floxin® otic is given for 10 days for otitis externa. The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. View information about the ndc, including active ingredients, administration.
8 rows manufacturers of ciprodex that have been granted an ndc (national drug. The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. Ciprodex® is dosed 7 days for all indications, while floxin® otic is given for 10 days for otitis externa. List of products in the national drug code with proprietary name ciprodex. View information about the ndc, including active ingredients, administration.
List of products in the national drug code with proprietary name ciprodex. The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. Ciprodex® is dosed 7 days for all indications, while floxin® otic is given for 10 days for otitis externa. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. View information about the ndc, including active ingredients, administration.
Ciprodex Coupons and Savings Guide Drug and
List of products in the national drug code with proprietary name ciprodex. View information about the ndc, including active ingredients, administration. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. The drug ciprodex contains a combination of these active pharmaceutical ingredients.
ciprodex 250 TAPharm
8 rows manufacturers of ciprodex that have been granted an ndc (national drug. The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. Ciprodex® is dosed 7 days for all indications, while floxin® otic is given for 10 days for otitis externa. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. List.
Do You Need Help With the Cost of Ciprodex? The RxSolution
8 rows manufacturers of ciprodex that have been granted an ndc (national drug. The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. List of products in the national drug code with proprietary name ciprodex. Ciprodex® is dosed 7 days for all indications,.
Ciprodex desktop items by Vincent Kwok at
Click the links below to download the 2025 valid and excluded icd diagnosis code lists. Ciprodex® is dosed 7 days for all indications, while floxin® otic is given for 10 days for otitis externa. The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. View.
Ciprodex FDA prescribing information, side effects and uses
List of products in the national drug code with proprietary name ciprodex. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. Ciprodex® is dosed 7 days for all indications, while floxin® otic is given for 10 days for otitis externa. View.
CIPRODEX Colirio 5mL BIOMED Belize
List of products in the national drug code with proprietary name ciprodex. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. View information about the ndc, including active ingredients, administration. Ciprodex® is dosed 7 days for all indications, while floxin® otic is given for 10 days for otitis externa. The drug ciprodex contains a.
Ciprodex Coupons and Savings Guide Drug and
Ciprodex® is dosed 7 days for all indications, while floxin® otic is given for 10 days for otitis externa. List of products in the national drug code with proprietary name ciprodex. Click the links below to download the 2025 valid and excluded icd diagnosis code lists. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. View.
Generic Ciprodex Ciprodex ear drops Ciprofloxacin for ear infections
Click the links below to download the 2025 valid and excluded icd diagnosis code lists. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. View information about the ndc, including active ingredients, administration. List of products in the national drug code with proprietary name.
Ciprodex Package Insert / Prescribing Information
The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. List of products in the national drug code with proprietary name ciprodex. View information about the ndc, including active ingredients, administration. Click the links below to download the 2025 valid and excluded icd diagnosis code.
Buy Ciprodex Otic Suspension online from Canada USA Script Helpers
The drug ciprodex contains a combination of these active pharmaceutical ingredients (apis):. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. View information about the ndc, including active ingredients, administration. List of products in the national drug code with proprietary name ciprodex. Click the links below to download the 2025 valid and excluded icd diagnosis code.
The Drug Ciprodex Contains A Combination Of These Active Pharmaceutical Ingredients (Apis):.
List of products in the national drug code with proprietary name ciprodex. View information about the ndc, including active ingredients, administration. 8 rows manufacturers of ciprodex that have been granted an ndc (national drug. Click the links below to download the 2025 valid and excluded icd diagnosis code lists.