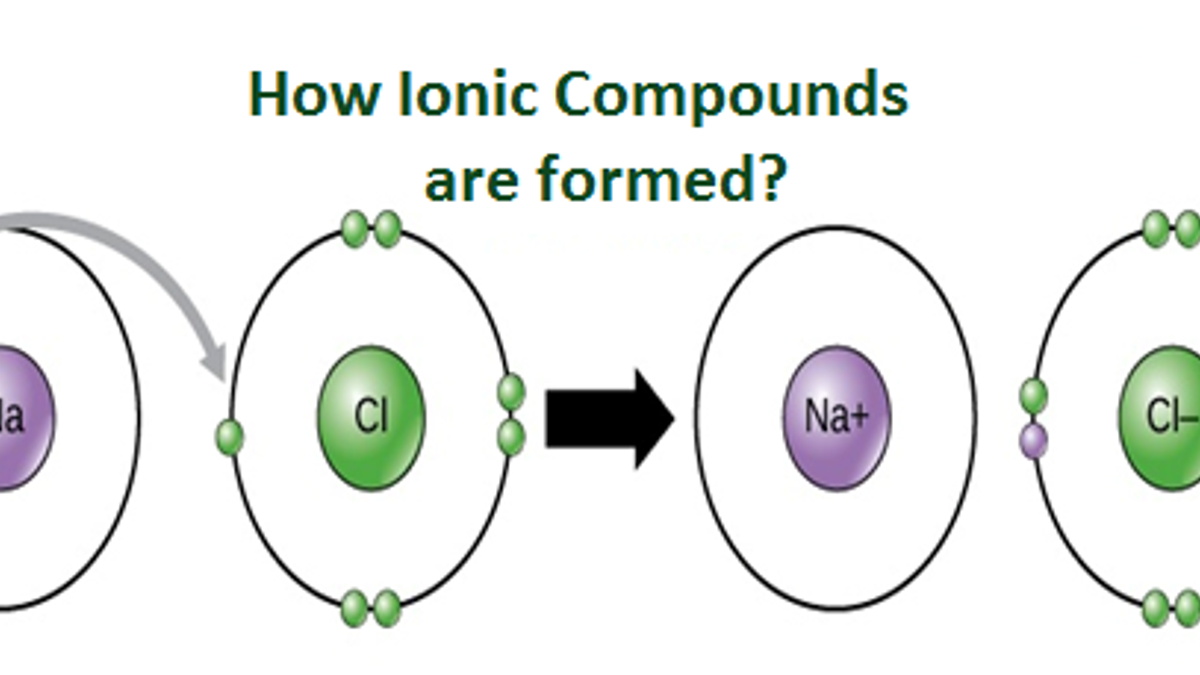
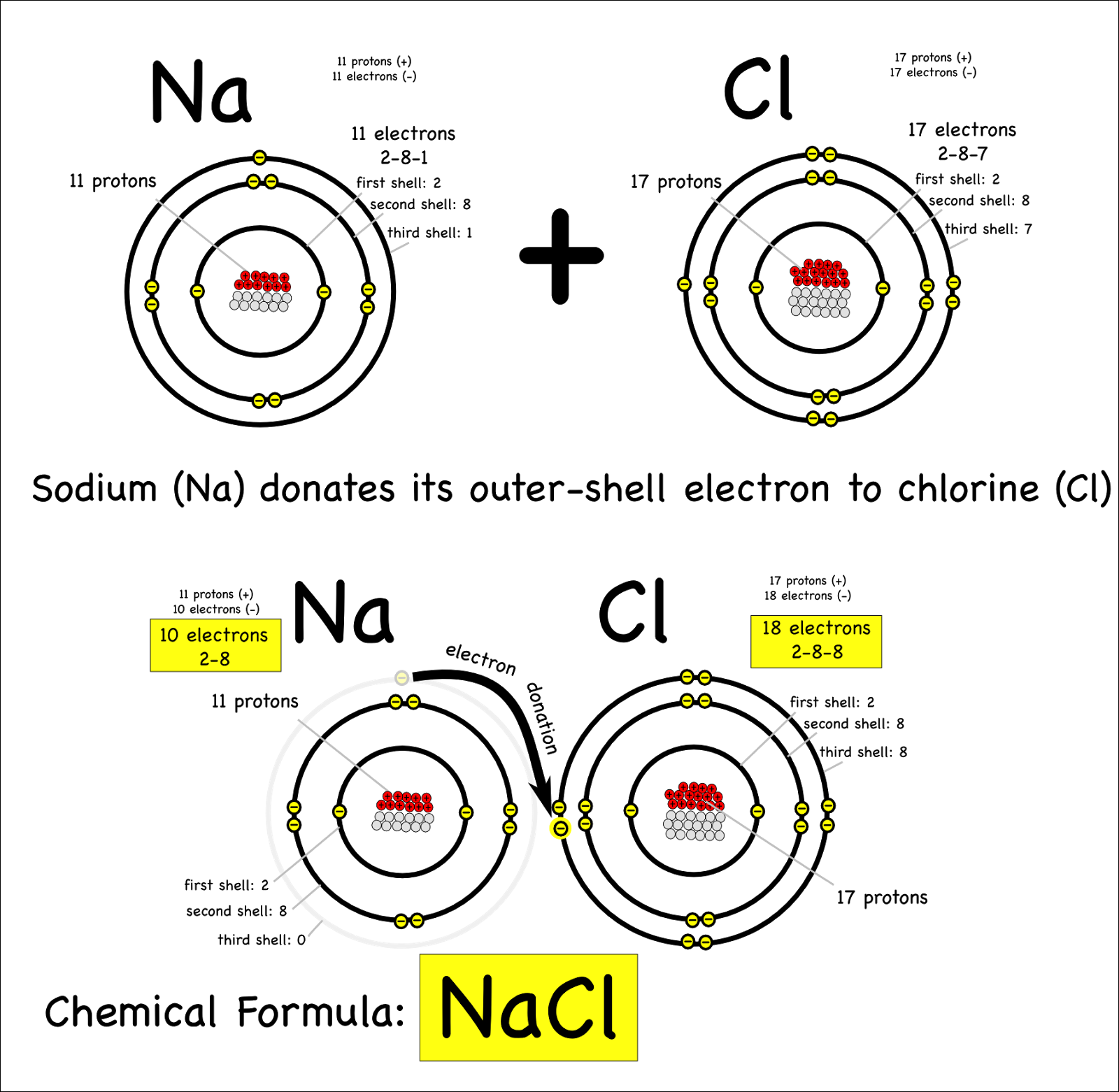
An Ionic Bond Forms Between A - Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a metal ion. Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative.
Ionic bonds are formed between cations and anions. Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a metal ion. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond.
Ionic bonding is the complete transfer of valence electron(s) between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion.
Examples of Ionic Bonds and Compounds
In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with.
Ionic Bond Examples Biology Dictionary
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonds are formed between cations and anions. Ionic bonding is the complete transfer of valence electron(s) between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a.
ionic bond Definition, Properties, Examples, & Facts Britannica
Ionic bonds are formed between cations and anions. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s).
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonds are formed between cations and anions. A cation is formed when a.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic bonding is the complete transfer of valence electron(s) between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonds are formed between cations and anions. A cation is formed when a.
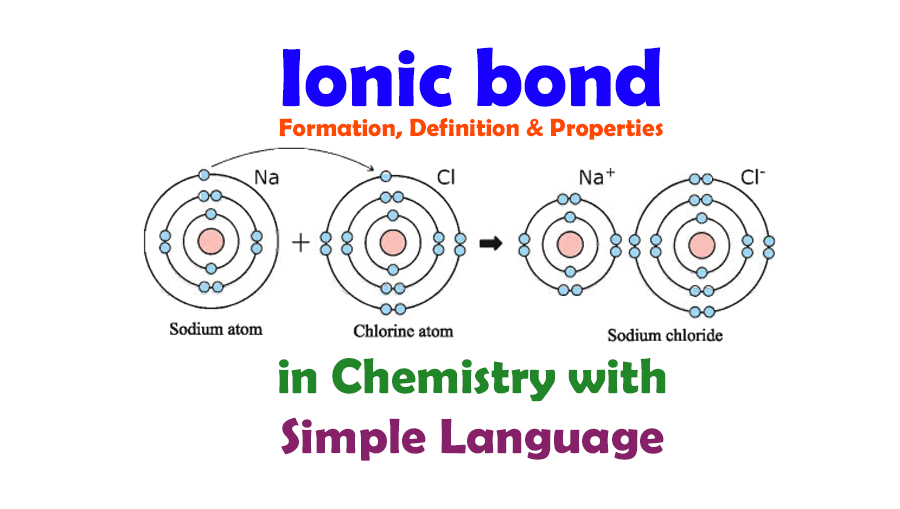
Ionic Bond and Ionic Bond Formation, Definition, Properties in
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s).
Diagram Ionic Bond
Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a.
Ionic Bond Facts, Definition, Properties, Examples,, 55 OFF
Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. A cation is formed when a.
Ionic Bonding Diagram
A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s) between atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations.
Diagram Of Ionic Bonding
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. A cation is formed when a metal ion. Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s).
An Ionic Bond Forms Between A ____ Ion With A Positive Charge And A ____ Ion With A Negative.
A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s) between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations and anions.
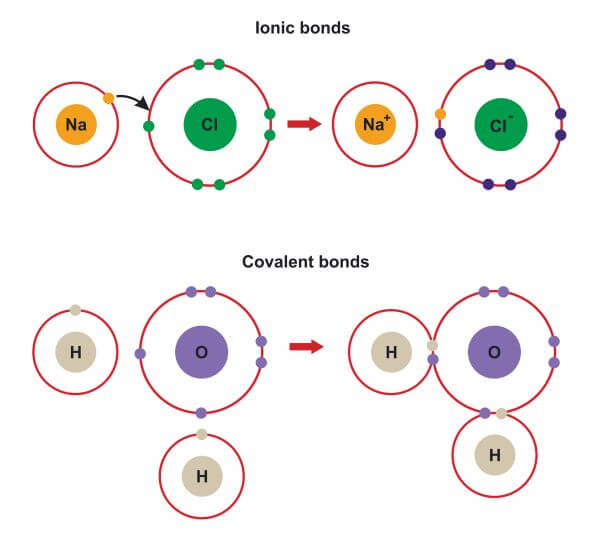


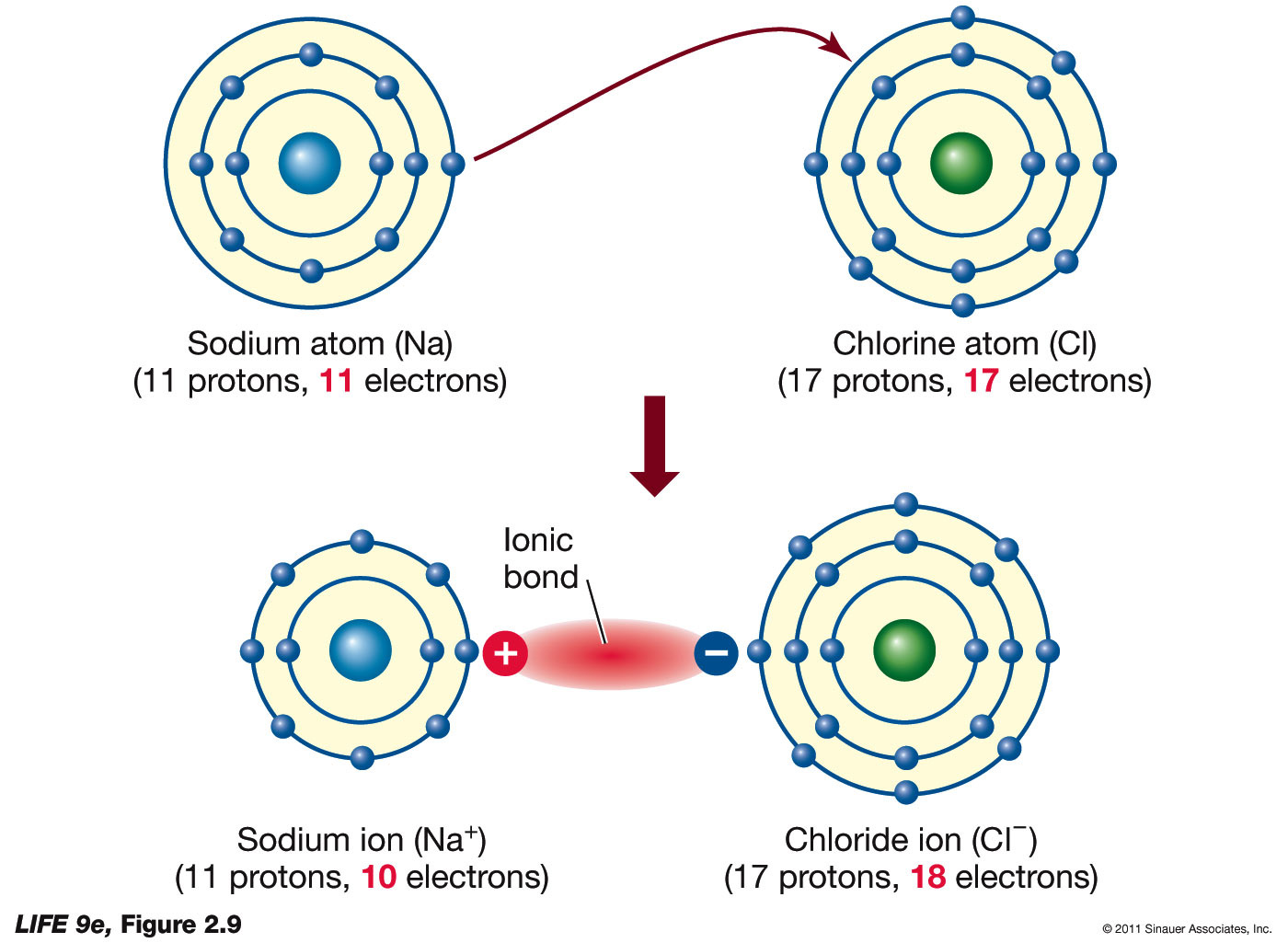
.PNG)